An overview of my PhD
Leading up to my PhD
There is surprisingly little known about what mitochondria do in fat. Mitochondria, “the powerhouse of the cell”, are in every cell of the body and ‘make’ energy. They are absolutely central to converting glucose and fats into energy. Fat tissue (‘adipose tissue’) is the main energy store of the body. Even lean individuals have enough calories stored in their fat to survive weeks. So, surely mitochondria are really important in fat tissue?
Until 2015 there was not much human genetic evidence for the importance of mitochondria in fat. Then, several research groups discovered that some patients with a condition affecting fat caused by a mutation in a mitochondrial gene (mitofusin 2 (MFN2)). This was interesting for a few reasons: the patients have fat loss on their legs but gain fat on their backs/necks, they have almost zero leptin (a hormone made by fat), and this condition only happened with one specific mutation in MFN2. Out of thousands of possible genetic changes in MFN2, this condition only came about when patients had two copies of the same mutation: Arginine → tryptophan at position 707.

From 2016 until 2022 I worked at the Institute of Metabolic Science at the University of Cambridge, in Prof. David Savage’s group. The severe insulin resistance service at the Institute looks after some patients with this Mfn2-related condition. Prior to me joining the group, the team had shown that this mutation in MFN2 causes fat cells in patients to become stressed and that the fat cells seemed to be making less leptin. (Leptin is a very important hormone – if people have a (very rare) genetic absence of leptin they develop extremely severe obesity at a young age.)
However, it was not known:
- If all cells in the body were stressed by this mutation, or just the fat cells.
- What the link is between Mfn2 and leptin.
- Why only this mutation in Mfn2 causes this condition, not other mutations in Mfn2.
These questions were the main aims of my PhD, which I started in December 2019.
What we did and found
Last month we published most of the results of my PhD in eLife. We used a mouse model to answer as many of these questions as possible.
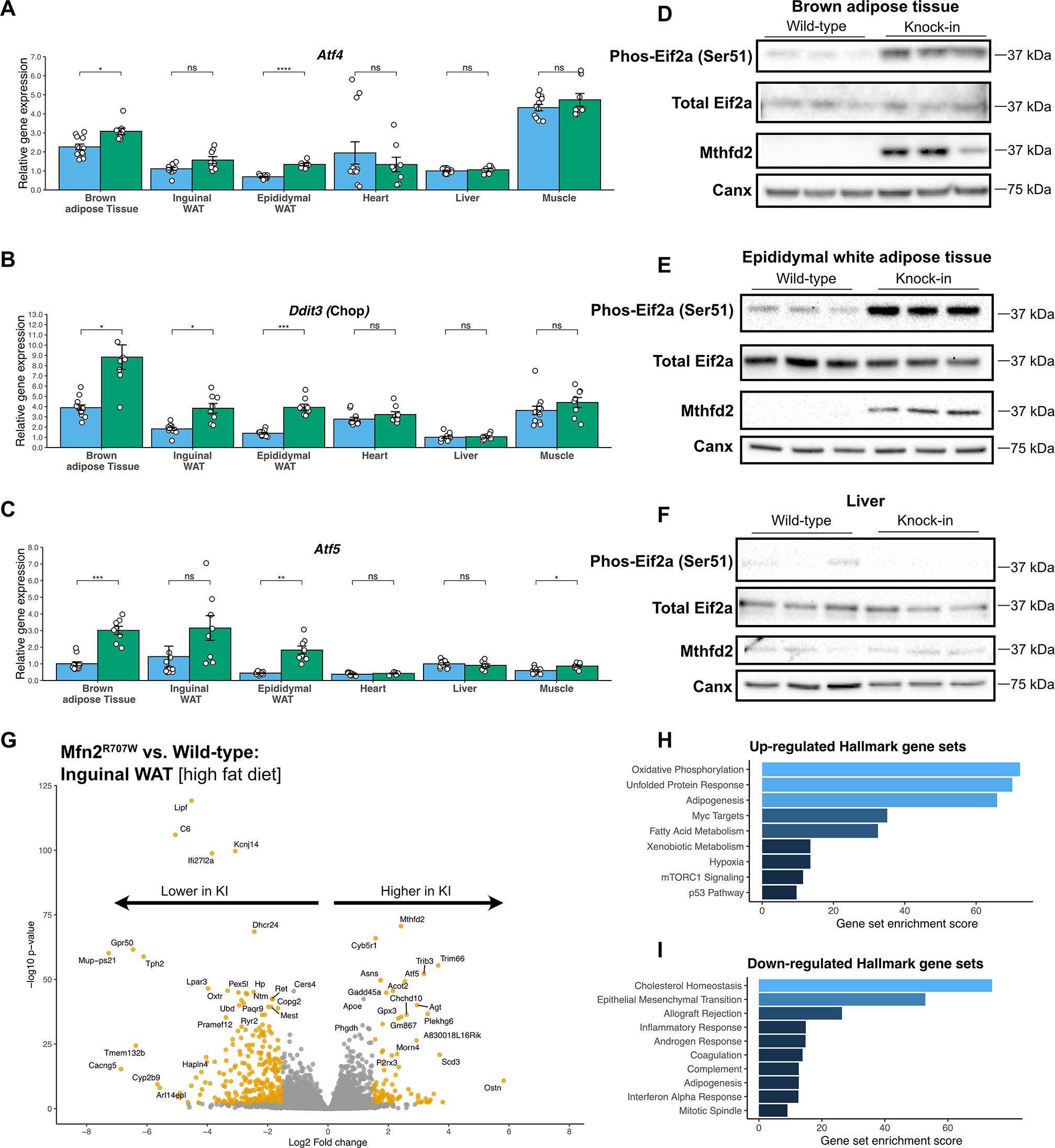
Prior to me arriving in the lab, others had generated mice that had the exact same mutation as the patients (i.e. Arginine → tryptophan at position 707 in the mouse mitofusin 2 gene (Mfn2)). We found that the mice make about the same amount of the mutant Mfn2 as the normal Mfn2 in every tissue we studied (fat, liver, muscle, heart); so the problem wasn’t just that the mutation caused less Mfn2.
Mitofusin 2 does what it says: it causes mitochondria to fuse. Next, we looked at mitochondria with an electron microscope. The mitochondria looked less fused from mice with the mutation, in fat tissue. This was only in fat tissue. The mitochondria looked normal in heart, muscle, and liver. Similarly, fat tissue from the mice showed the same ‘stress signals’ that the patients had. Liver, muscle, and heart didn’t show any stress signals. This tells us that the mitofusin mutation causes cellular stress and only seems to affect fat.
But it didn’t seem to affect mice like it did patients. The patients had type 2 diabetes and fatty liver, as well as fat overgrowth. We looked very carefully and couldn’t find any obvious differences in the mice.

Except the mice did have lower leptin, just like the patients. The leptin wasn’t low enough to make the mice obese. We actually found several other fat hormones to be low, too. We also showed that stressing fat cells in a dish causes them to make less leptin.
All together, this shows that:
- This mutation in Mfn2 only affects fat cells and causes them to be stressed.
- Stressed fat cells make less of all fat hormones (including leptin)
We tried several ways to explain why this mutation only affects fat, but we couldn’t reach a conclusion before my PhD ended. The team will carry on working to try and figure that out.
Why this matters
We have provided further evidence for specific genes (dys)regulating the function of fat via mitochondria: a connection between mitochondria (that make energy) and the body’s main energy store.
Also this is a clear link between mitochondria → stress in fat tissue → fat hormone levels. We know that some people (without any major gene mutations) have lower levels of leptin, and perhaps this pathway is important.
The mitofusin genes are important in other fields, including cancer and neurology. This work contributes to the knowledge base.
——-
I will be forever grateful for everyone who taught and helped me in the IMS (and beyond) over the last 7 years contributing to this project.
My full thesis is also available and there is another paper that is still being revised, but is available in a preliminary form. RNAseq data and code used is also freely available.